Theory bond valence chapter ppt powerpoint presentation px pz py hybrid sp3 equivalent orbitals called each Theory valence vbt vb chemistry limitations postulates electron Valence bonding vbt chemical
PPT - Valence bond theory PowerPoint Presentation, free download - ID
Orbitals bond valence theory bonding atomic covalent sp chemistry oxygen hybrid structure electron two bonds organic mcc hydrogen libretexts draw
Theory bond valence geometry
Valence bond theoryValence bond coordination compound Valence bond theory hybrid orbitals atomicBond valence ionic bonding covalent.
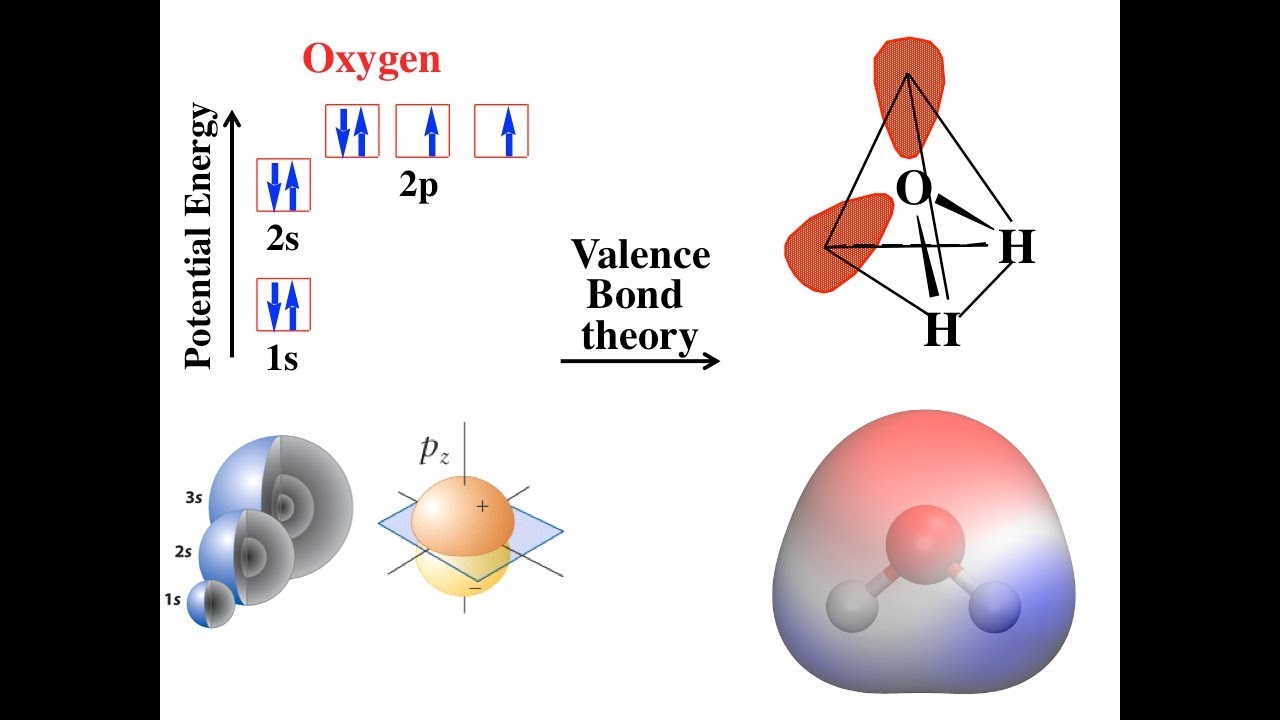
Bond valence orbitals atomsValence sigma bonds pi acetylene ch2 h2c electrons Valence bond theoryValence bond theory probes fundamental nature of hydrogen bonding.

Theory bond valence sigma bonds pi vbt vb orbital bonding molecular limitations formation etoosindia chemistry
Valence bond theory electron configurationValence ch4 sp3 contains electron Theory vsepr bond valence between difference comparison definition hybridization piTheory bond valence bonding orbital overlap focus let chapter formed ppt powerpoint presentation earlier atoms saw single between two.
Valence bond theoryDifference between vsepr and valence bond theory Bonding sigma bond between interaction two orbitals covalent organic atoms valence theory repulsion pi repulsive atomic nuclei chemistry mcc positiveBond hydrogen bonding valence theory chemistry.
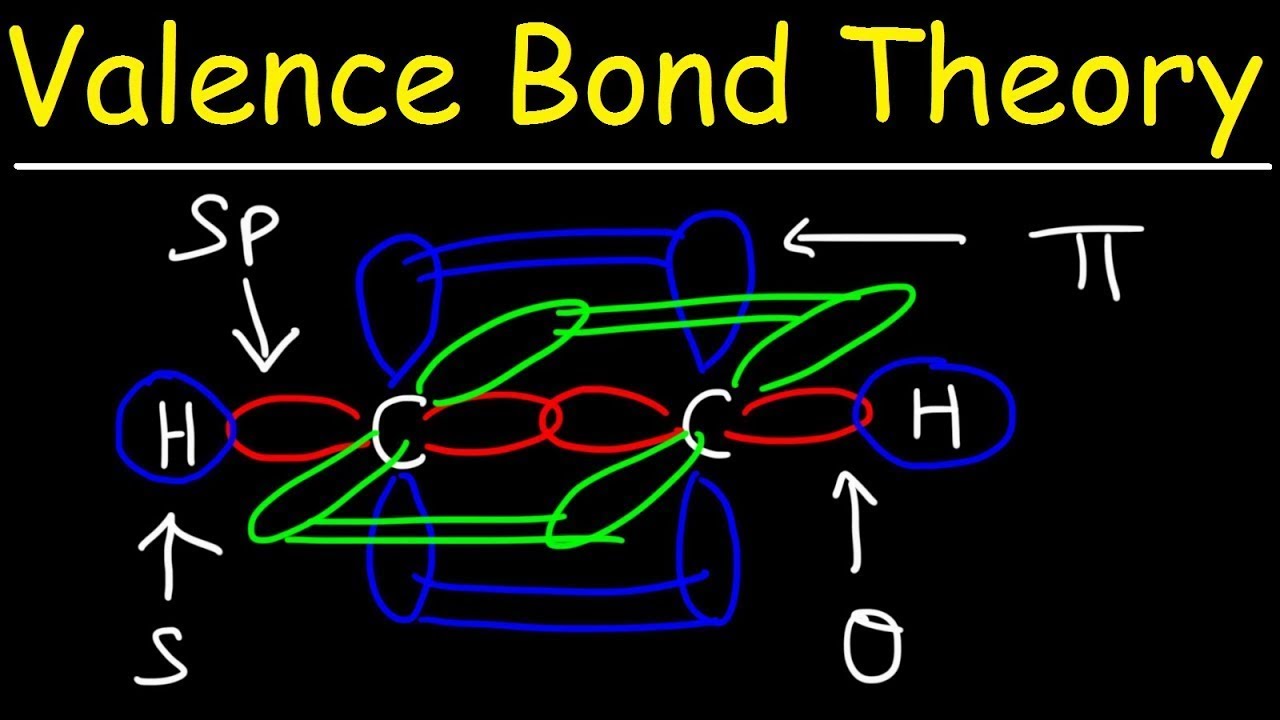
Theory bond valence vsepr vbt ppt powerpoint presentation bonding
Valence bond theory pptxValence bond theory pptx Covalent bond valence hcl bonding theory theories overlap spin opposite electrons ppt powerpoint presentation cl orbitalsValence bond theory.
Valence bond (vb) theoryTheory bond valence vbt ppt chapter orbital bonding theories powerpoint presentation orbitals two overlap molecular Valence bond theoryValence theory bond.

Hybridisation theory orbital orbitals valence bonding chem atom
Valence bond theory class 11 chemistry (vbt )Bond valence theory chapter presentation ppt molecules diatomic slide1 slideserve Valence bond theory & hybrid atomic orbitals.
.